Molecular orbitals pi butadiene diagram mo draw energy two frontier lowest following which highlighted ve
Table of Contents
Table of Contents
If you’re studying chemistry, you’ve likely heard the term “frontier orbitals” thrown around. Drawing them can be confusing and overwhelming, but fear not! In this post, we’ll break down the steps to drawing frontier orbitals in a way that’s easy to understand.
The Struggle of Drawing Frontier Orbitals
As a chemistry student, you’re expected to have a deep understanding of molecular orbitals and how they interact with one another. It can be frustrating when trying to visualize these structures in your head or draw them out on paper for an exam. Frontier orbitals are especially difficult because they are involved in chemical reactions, making understanding their structure and behavior critical to success in the field.
How to Draw Frontier Orbitals
First, let’s define what a frontier orbital is. In chemistry, a frontier orbital refers to the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of a molecule. The HOMO and LUMO are critical for understanding molecular reactivity and bonding within a system.
To draw frontier orbitals, you first need to determine the HOMO and LUMO of the molecule. This can be done using computational software, like Gaussian or Molpro. Once you have determined the HOMO and LUMO, you can use that information to construct the corresponding frontier orbitals.
The HOMO is drawn as a filled-in orbital with electrons, while the LUMO is drawn as an empty orbital with no electrons. The HOMO and LUMO are often drawn side-by-side to show the energy difference between them. The HOMO is typically shown at a higher energy level than the LUMO.
It’s important to note that not all frontier orbitals are the same. There are different types, including sigma (σ) and pi (π) orbitals, and they can have various shapes and sizes. It’s crucial to have a strong foundation in molecular orbital theory to understand the differences and how to draw them.
Going Deeper into Drawing Frontier Orbitals
When learning how to draw frontier orbitals, it can be helpful to have visual aids. Below are a few images of frontier orbitals and what they look like:
 When drawing frontier orbitals, it’s important to pay attention to the molecular orbitals of the system. Frontier orbitals are involved in many critical processes, making them a fundamental concept within the field of chemistry.
When drawing frontier orbitals, it’s important to pay attention to the molecular orbitals of the system. Frontier orbitals are involved in many critical processes, making them a fundamental concept within the field of chemistry.
Important Tips for Drawing Frontier Orbitals
Here are some key things to keep in mind when drawing frontier orbitals:
- Start by determining the HOMO and LUMO of the molecule
- Draw the HOMO as a filled-in orbital with electrons
- Draw the LUMO as an empty orbital with no electrons
- Pay attention to the type of frontier orbital you are drawing (sigma or pi)
- Make sure you have a solid foundation in molecular orbital theory to understand the structure and behavior of frontier orbitals
Personal Experience with Drawing Frontier Orbitals
When I first started studying chemistry, drawing frontier orbitals was intimidating. It wasn’t until I put in the time to understand molecular orbital theory that I began to grasp the concept. Having visual aids, like images and diagrams, helped me visualize the orbitals and gain a better understanding of their behavior.
Practice Makes Perfect
Like with most things in chemistry, practice makes perfect. Drawing frontier orbitals can be challenging at first, but with time and effort, you can master this essential concept. Don’t be afraid to ask questions and seek out additional resources to aid in your understanding.
Question and Answer
Q: Why are frontier orbitals important?
A: Frontier orbitals are critical for understanding chemical reactions and molecular interactions. Knowing the HOMO and LUMO of a molecule can provide insight into its reactivity and bonding potential.
Q: How do you know if you’re drawing a sigma or pi orbital?
A: Sigma orbitals are typically formed by head-to-head or tail-to-tail overlap of atomic orbitals, while pi orbitals are formed by sideways overlap of atomic orbitals.
Q: Can you draw multiple types of frontier orbitals for one molecule?
A: Yes, it’s possible to have multiple types of frontier orbitals within a single molecule.
Q: What is the difference between the HOMO and the LUMO?
A: The HOMO is the highest occupied molecular orbital, while the LUMO is the lowest unoccupied molecular orbital.
Conclusion of How to Draw Frontier Orbitals
Drawing frontier orbitals can be challenging, but it’s essential for understanding chemistry at a molecular level. To master this skill, you need a solid foundation in molecular orbital theory and a willingness to practice. By following the tips outlined in this post, you can better grasp this vital concept and succeed in your chemistry studies.
Gallery
The Pi Molecular Orbitals Of Butadiene And How To Draw Them

Photo Credit by: bing.com / molecular orbitals pi butadiene diagram mo draw energy two frontier lowest following which highlighted ve
The Pi Molecular Orbitals Of Benzene – Master Organic Chemistry
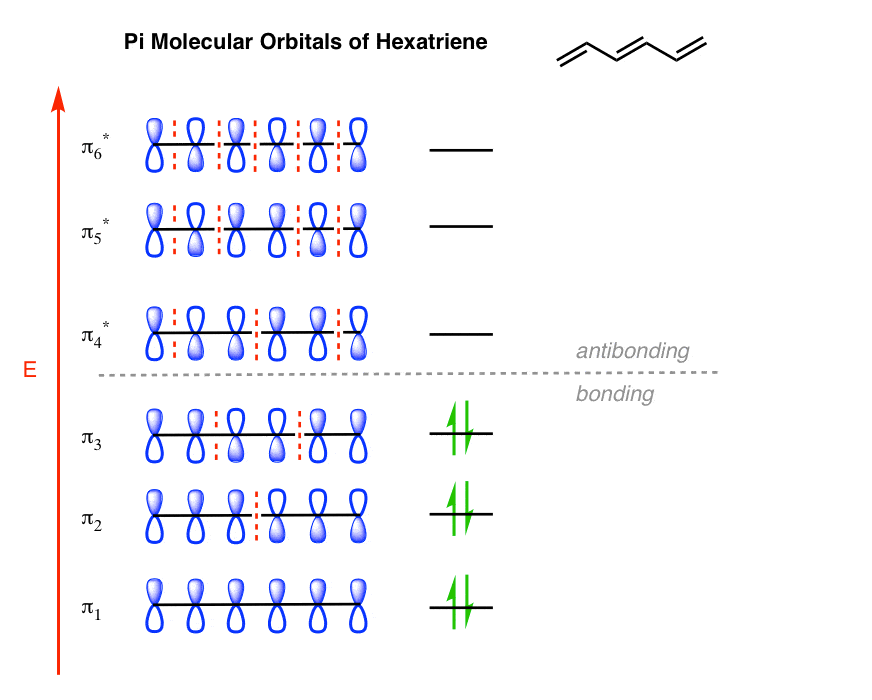
Photo Credit by: bing.com / benzene orbital orbitals chemistry
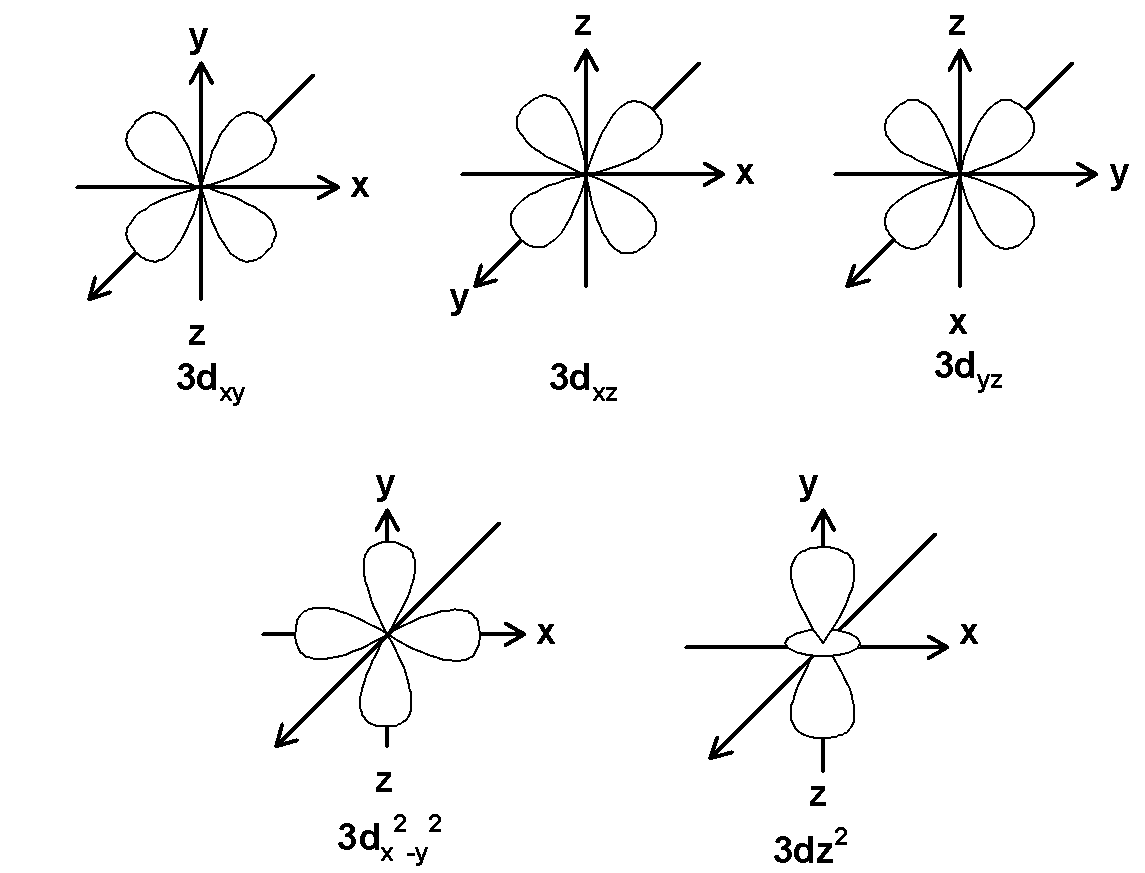
What Is An Orbital? Draw The Shapes Of The 1s, 2s, $\\text 2{{\\text

Photo Credit by: bing.com / orbitals orbital 2s
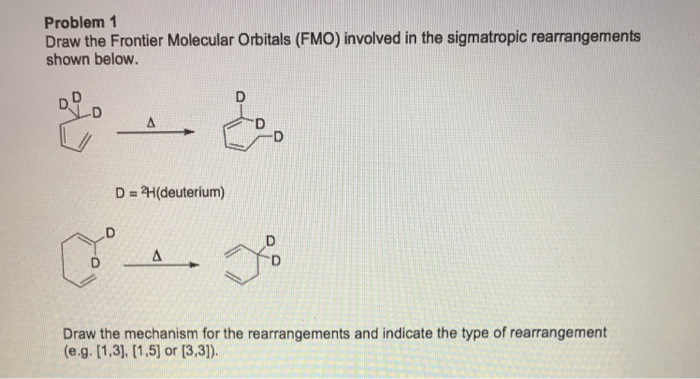
Solved: Draw The Frontier Molecular Orbitals (FMO) Involve… | Chegg.com
Photo Credit by: bing.com / draw frontier fmo orbitals molecular rearrangements sigmatropic involved shown problem below mechanism solved transcribed text rearrangement indicate type
Calculated Frontier Orbitals For Geometry 4b. | Download Scientific Diagram

Photo Credit by: bing.com / orbitals