Orbitals atomic orbital theory shapes chemistry iv visionlearning chemwiki uc davis beginning complex figure
Table of Contents
Table of Contents
A picture is worth a thousand words, and this is especially true when it comes to drawing d orbitals in chemistry. Whether you’re a student just starting out or a seasoned chemist, the ability to draw d orbitals is essential. Not only does it help you visualize the electrons in an atom, but it also allows you to predict the atom’s behavior in chemical reactions.
Pain Points
However, many students struggle with the concept of how to draw d orbitals. For one thing, it can be difficult to visualize a three-dimensional structure on a flat piece of paper. Additionally, some students may not understand the principles behind d orbitals and why they are important.
How to Draw d Orbitals
So, how do you draw d orbitals? The first step is understanding the principles behind orbitals. Essentially, electrons in atoms occupy spaces called orbitals, which are defined by their energy level and shape. Each orbital can hold up to two electrons, and the electrons “spin” in opposite directions to maintain stability.
When it comes to d orbitals, there are five possible shapes: d(x^2-y^2), d(xy), d(xz), d(yz), and d(z^2). Each shape represents a different orientation in space, and it is important to be able to visualize each one in 3D. To draw d orbitals, start with a coordinate plane (x, y, z) and use the equations for each shape to plot the points. Then, connect the points to create the boundary surface of the orbital.
Summary
In summary, drawing d orbitals requires an understanding of the principles behind orbitals and the ability to visualize in 3D. By mastering these skills, you can better understand the behavior of atoms in chemical reactions and predict how they will interact.
How to Draw d Orbitals: Tips and Tricks
When I first started learning how to draw d orbitals, I found it helpful to use physical models. By constructing a 3D model with plasticine or similar materials, I was able to better understand the orbital’s shape and orientation. Additionally, I found it helpful to practice drawing the orbitals on graph paper before moving on to more complex models.
 Understanding the Shapes of d Orbitals
Understanding the Shapes of d Orbitals
One of the most important concepts to understand when it comes to drawing d orbitals is the principle of symmetry. Each orbital has a specific symmetry that is determined by its shape and orientation. By understanding this principle, you can better predict how the orbital will behave in chemical reactions.
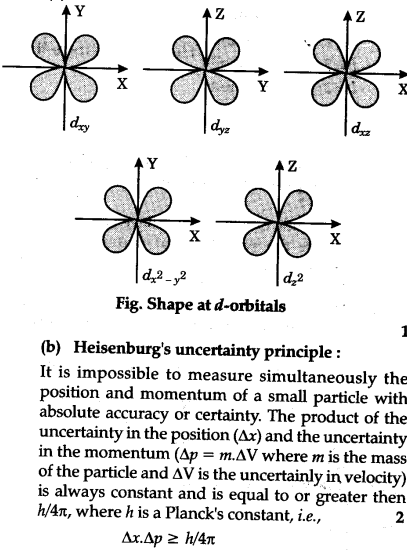 ### The Importance of d Orbitals in Chemistry
### The Importance of d Orbitals in Chemistry
So why are d orbitals so important in chemistry? One reason is that they determine the electronic structure of transition metals, which are essential in many biological and industrial processes. Additionally, d orbitals can help us understand the color and magnetic properties of metals, as well as their reactivity in chemical reactions.
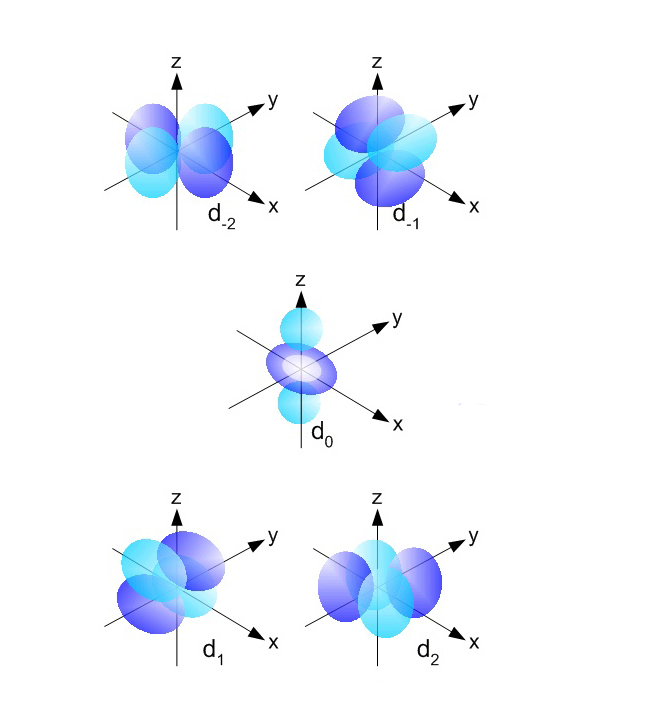 #### Tips for Mastering the Art of Drawing d Orbitals
#### Tips for Mastering the Art of Drawing d Orbitals
You may find it helpful to practice drawing d orbitals with different equations to gain a deeper understanding of the shapes and orientations. Additionally, it can be helpful to use computer programs or online tools to create 3D models of d orbitals.
Question and Answer
Q: Why are d orbitals important to understand in chemistry?
A: D orbitals are important because they determine the electronic structure of transition metals, which are essential in many biological and industrial processes.
Q: How many electrons can a d orbital hold?
A: A d orbital can hold up to two electrons.
Q: What is the principle of symmetry?
A: The principle of symmetry refers to the fact that each orbital has a specific symmetry that is determined by its shape and orientation.
Q: Are there any tips for mastering the art of drawing d orbitals?
A: Yes, it can be helpful to practice drawing d orbitals with different equations and to use computer programs or online tools to create 3D models.
Conclusion of how to draw d orbitals
Mastering the art of drawing d orbitals requires patience, practice, and a willingness to learn. By understanding the principles behind orbitals and the importance of d orbitals in chemistry, you can better understand the world around you and make more accurate predictions about chemical reactions.
Gallery
File:D Orbitals.png - Wikimedia Commons

Photo Credit by: bing.com / orbitals
Chemistry - Orbitals
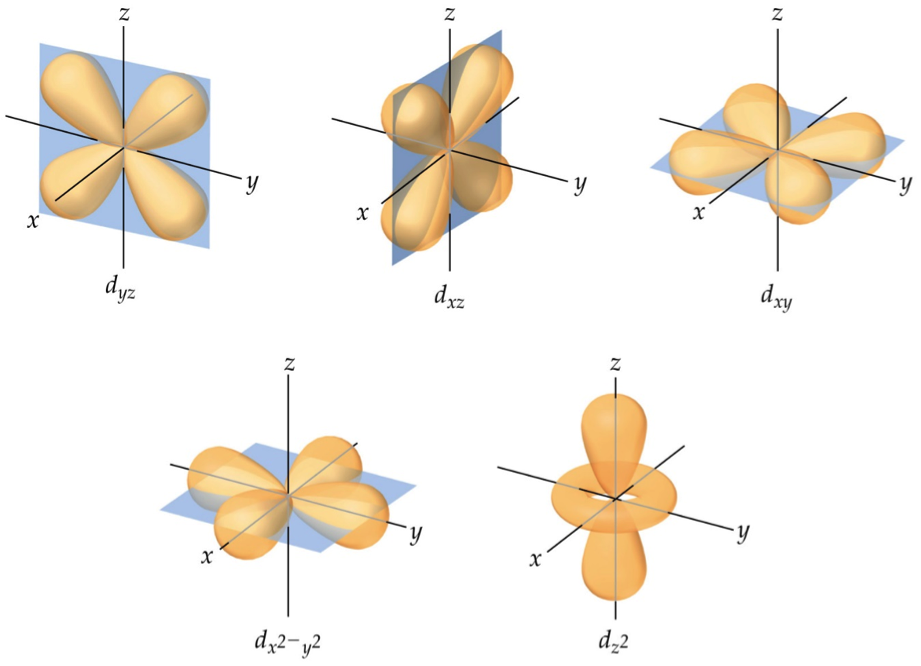
Photo Credit by: bing.com / orbitals orbital chemistry electrons upwards quoracdn qph
Atomic Theory IV | Chemistry | Visionlearning

Photo Credit by: bing.com / orbitals atomic orbital theory shapes chemistry iv visionlearning chemwiki uc davis beginning complex figure
Draw The Shapes Of D-orbitals - CBSE Class 11 Chemistry - Learn CBSE Forum

Photo Credit by: bing.com / orbitals shapes draw kb
Draw The Boundary Surface Diagram For D-orbital - 10275423
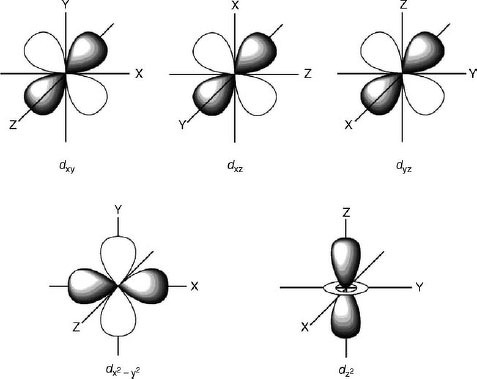
Photo Credit by: bing.com / orbital